In, and, cracking is the process whereby complex such as or long-chain are broken down into simpler molecules such as light hydrocarbons, by the breaking of -carbon in the precursors. The of cracking and the end products are strongly dependent on the and presence of. Cracking is the breakdown of a large into smaller, more useful. Simply put, hydrocarbon cracking is the process of breaking a long-chain of hydrocarbons into short ones.
FlexiSign Pro 10.5 Free Download for Windows supporting both architectures i.e. 32 bit and 64 bit. Setup file is completely standalone and also its an offline installer. FlexiSIGN-PRO 7.6 is free to download from our software library. Our built-in antivirus checked this download and rated it as virus free. The program's installer files are generally known as App.exe or issch.exe etc. The most popular versions of the FlexiSIGN-PRO 8.1, 7.6 and 7.5. Flexisign pro 76v2 software free download.
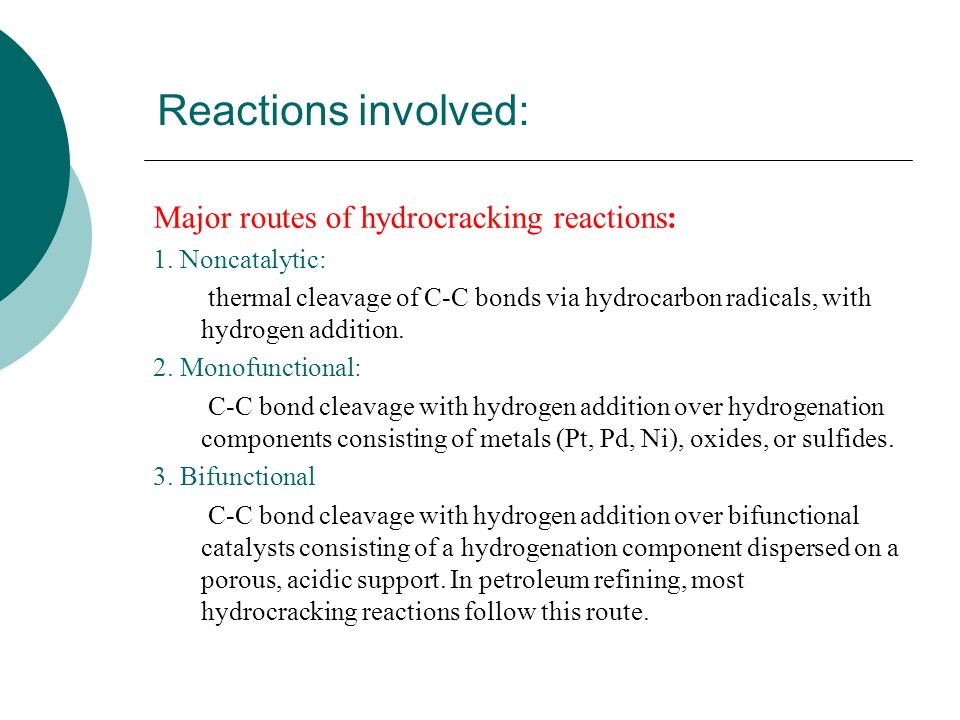
Jul 6, 2014 - The advantages of hydrocracking include its ability to handle a wide range of feedstock, as well as the selectivity of its distillates. The primary objective of both cracking processes is to produce lighter saturated hydrocarbons with reduced molecular weights and boiling points from heavy oils. For technical reasons, hydrocracking was only made possible in the late Fifties. Of hydrocarbon) with the advantage that impurities, such as sulfur and nitrogen. Hydrocracking does not generate any alkenes because hydrogen reacts with. Free download game monopoly modoo marble terbaru full version hd.

This process requires high temperatures and high pressure. More loosely, outside the field of petroleum chemistry, the term 'cracking' is used to describe any type of splitting of molecules under the influence of heat, catalysts and solvents, such as in processes of. Fluid catalytic cracking produces a high yield of and, while hydrocracking is a major source of,,, and again yields LPG. Contents • • • • • • • • • • • • • History and patents [ ] Among several variants of thermal cracking methods (variously known as the ', ', 'Burton-Humphreys cracking process', and 'Dubbs cracking process'), a Russian engineer, invented and patented the first in 1891 (Russian Empire, patent no. 12926, November 7, 1891). One installation was used to a limited extent in Russia, but development was not followed up.
In the first decade of the 20th century the American engineers and Robert E. Humphreys independently developed and patented a similar process as U.S. Patent 1,049,667 on June 8, 1908.
Among its advantages was the fact that both the condenser and the boiler were continuously kept under pressure. In its earlier versions it was a batch process, rather than continuous, and many patents were to follow in the USA and Europe, though not all were practical.
In 1924, a delegation from the American visited Shukhov. Sinclair Oil apparently wished to suggest that the patent of Burton and Humphreys, in use by Standard Oil, was derived from Shukhov's patent for oil cracking, as described in the Russian patent.
If that could be established, it could strengthen the hand of rival American companies wishing to invalidate the Burton-Humphreys patent. In the event Shukhov satisfied the Americans that in principle Burton's method closely resembled his 1891 patents, though his own interest in the matter was primarily to establish that 'the Russian oil industry could easily build a cracking apparatus according to any of the described systems without being accused by the Americans of borrowing for free'. At that time, just a few years after the, Russia was desperate to develop industry and earn foreign exchange, so their oil industry eventually did obtain much of their technology from foreign companies, largely American. At about that time, was being explored and developed and soon replaced most of the purely thermal cracking processes in the fossil fuel processing industry. The replacement was not complete; many types of cracking, including pure thermal cracking, still are in use, depending on the nature of the feedstock and the products required to satisfy market demands. Thermal cracking remains important, for example in producing naphtha, gas oil, and coke, and more sophisticated forms of thermal cracking have been developed for various purposes. These include,,.
Cracking methodologies [ ] Thermal methods [ ] Thermal cracking was the first category of hydrocarbon cracking to be developed. Thermal cracking is an example of a reaction whose energetics are dominated by (∆S°) rather than by (∆H°) in the Gibbs Free Energy equation ∆G°=∆H°-T∆S°. Although the bond dissociation energy D for a carbon-carbon single bond is relatively high (about 375 kJ/mol) and cracking is highly endothermic, the large positive entropy change resulting from the fragmentation of one large molecule into several smaller pieces, together with the extremely high temperature, makes T∆S° term larger than the ∆H° term, thereby favoring the cracking reaction. [ ] [ – ] Thermal cracking [ ] Modern high-pressure thermal cracking operates at absolute pressures of about 7,000 kPa. An overall process of disproportionation can be observed, where 'light', hydrogen-rich products are formed at the expense of heavier molecules which condense and are depleted of hydrogen. The actual reaction is known as and produces, which are the basis for the economically important production of.
[ ] Thermal cracking is currently used to 'upgrade' very heavy fractions or to produce light fractions or distillates, burner fuel and/or. Two extremes of the thermal cracking in terms of product range are represented by the high-temperature process called 'steam cracking' or (ca. 750 °C to 900 °C or higher) which produces valuable and other feedstocks for the petrochemical industry, and the milder-temperature (ca. 500 °C) which can produce, under the right conditions, valuable, a highly crystalline petroleum coke used in the production of for the and industries. [ ] developed one of the earliest thermal cracking processes in 1912 which operated at 700–750 °F (371–399 °C) and an absolute pressure of 90 psi (620 kPa) and was known as the. Shortly thereafter, in 1921,, an employee of the Company, developed a somewhat more advanced thermal cracking process which operated at 750–860 °F (399–460 °C) and was known as the.